First liver cancer patient treated with microspheres irradiated in TU Delft
Radboud Translational Medicine will perform the dispensing of below product.
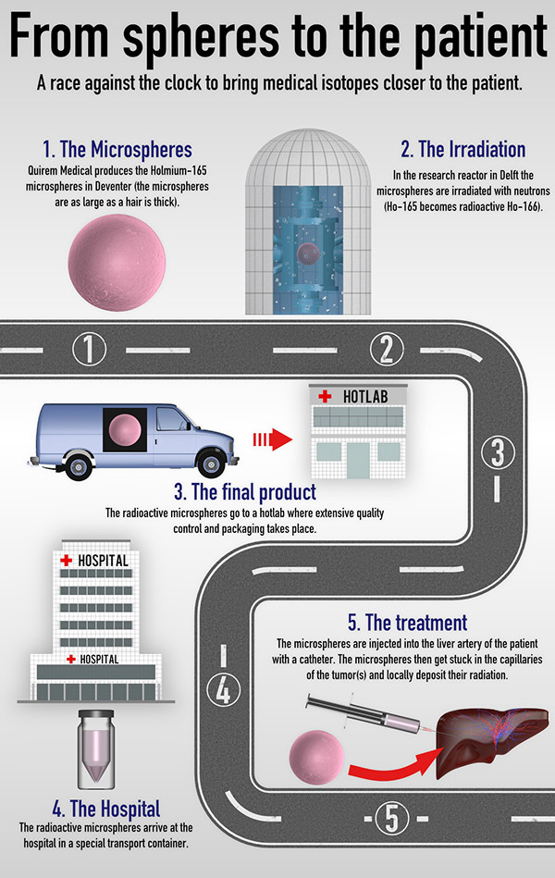
The production and distribution of short-lived medical isotopes is a race against time. To be able to get medical isotopes with the required level of radioactivity to the patient, TU Delft researchers have been working closely with Quirem Medical and Radboudumc*. Today, in Italy, the first liver cancer patient will be treated with special radioactive microspheres that were produced in Delft. This innovative liver cancer treatment is conducted using tiny spheres – about the thickness of a hair – that are packed with the radioisotope Holmium-166. The microspheres are activated in a new flexible irradiation facility that was recently developed by TU Delft’s Reactor Institute Delft (RID).
A special new sample holder has been designed for this research reactor in which Holmium-165 is irradiated. The new sample holder allows the spheres to be irradiated with a large amount of radioactivity without damaging the biodegradable envelope with gamma radiation. The upshot is a new smart method for converting Holmium-165 into radioactive Holmium-166. The main advantages are that the microspheres can be loaded with more radioactivity and there is a longer window of time to get the end product in the hospital where it can be administered to the patient. Thanks to this TU Delft invention, patients around the world will now be able to benefit from this internal radiotherapy treatment.
The principle of an adjustable irradiation chamber can be applied to any nuclear reactor. "This has brought the production of isotopes for medical applications closer to the patients," explains TU Delft researcher Dr Antonia Denkova, head of the research project. You can read more about the flexible irradiation facility in ‘A flexible irradiation facility for producing radioisotopes’.
* Researchers at Radboudumc are studying new applications of the microspheres for liver, brain, head and neck, and pancreatic tumours.
Contact
dr. ir. Antonia Denkova (onderzoeksleider TU Delft), +31(0)15 2784471
dr. Frank Nijsen (onderzoeker Radboudumc), +31(0)6 40675781
Claire Hallewas (persvoorlichter TU Delft),+31(0)6 40953085
Other news

18F-FDOPA now available
Fluorodopa (18F) is now available for clinical studies and - with doctor's certificate - for routine...

Huntington’s Disease: A Review of the Known PET Imaging Biomarkers and Targeting Radiotracers
Klaudia Cybulska (postdoc radiochemistry) has written an article about Huntington's Disease. Abstra...

Production of novel diagnostic radionuclides in small medical cyclotrons
Employees from RTM and Radboudumc have published a new article: 'Production of novel diagnostic radi...
First Patient Dosed with TLX250-CDx in Italy for ccRCC
The below product is manufactured at RTM First Patient dosed with TLX250-CDx in Italy for ccRCC Th...
Interested in a collaboration?
Contact us to discuss the possibilities.





