First liver cancer patient treated with microspheres irradiated in TU Delft
Radboud Translational Medicine will perform the dispensing of below product.
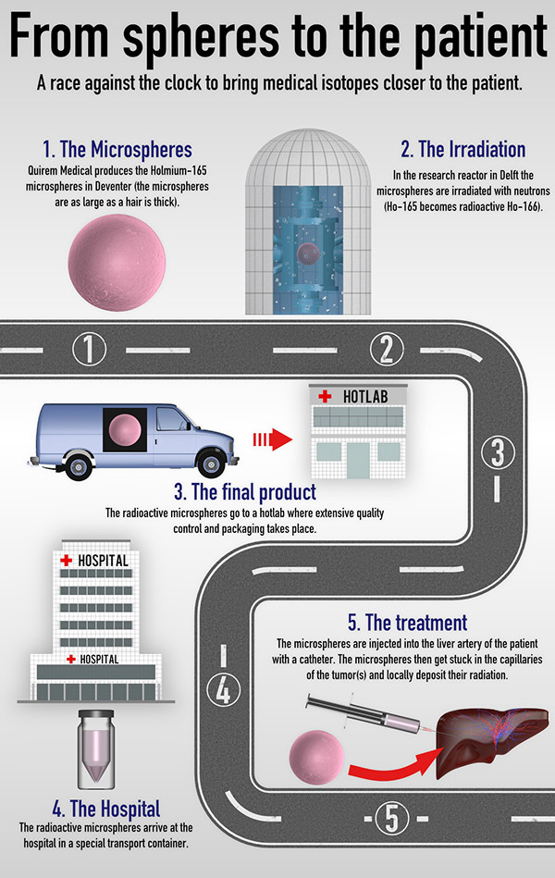
The production and distribution of short-lived medical isotopes is a race against time. To be able to get medical isotopes with the required level of radioactivity to the patient, TU Delft researchers have been working closely with Quirem Medical and Radboudumc*. Today, in Italy, the first liver cancer patient will be treated with special radioactive microspheres that were produced in Delft. This innovative liver cancer treatment is conducted using tiny spheres – about the thickness of a hair – that are packed with the radioisotope Holmium-166. The microspheres are activated in a new flexible irradiation facility that was recently developed by TU Delft’s Reactor Institute Delft (RID).
A special new sample holder has been designed for this research reactor in which Holmium-165 is irradiated. The new sample holder allows the spheres to be irradiated with a large amount of radioactivity without damaging the biodegradable envelope with gamma radiation. The upshot is a new smart method for converting Holmium-165 into radioactive Holmium-166. The main advantages are that the microspheres can be loaded with more radioactivity and there is a longer window of time to get the end product in the hospital where it can be administered to the patient. Thanks to this TU Delft invention, patients around the world will now be able to benefit from this internal radiotherapy treatment.
The principle of an adjustable irradiation chamber can be applied to any nuclear reactor. "This has brought the production of isotopes for medical applications closer to the patients," explains TU Delft researcher Dr Antonia Denkova, head of the research project. You can read more about the flexible irradiation facility in ‘A flexible irradiation facility for producing radioisotopes’.
* Researchers at Radboudumc are studying new applications of the microspheres for liver, brain, head and neck, and pancreatic tumours.
Contact
dr. ir. Antonia Denkova (onderzoeksleider TU Delft), +31(0)15 2784471
dr. Frank Nijsen (onderzoeker Radboudumc), +31(0)6 40675781
Claire Hallewas (persvoorlichter TU Delft),+31(0)6 40953085
Other news

RTM added as distributor NeuraCeq®
Exciting news! Life Molecular Imaging’s NeuraCeq® 300 MBq/ml solution for injection (florbetaben 18F...

RTM receives marketing authorisation for Fludeoxyglucose (18F)
We are happy to announce that RTM has received marketing authorization for 18F-FDG of 17 July 2018....

Upcoming event
From October 15 to October 19, Radboud translational medicine will attend the 29th annual congress o...
First Patient Dosed in Phase II Study of TLX250-CDx in Triple-Negative Breast Cancer
The below product is manufactured at RTM First Patient Dosed in Phase II Study of TLX250-CDx in Tri...
Interested in a collaboration?
Contact us to discuss the possibilities.





