Radboud Translational Medicine’s young team consists of enthusiastic employees who have earned their stripes in the (radio)pharmaceutical industry, science and medical imaging. Together, we are committed to the development of radiopharmaceuticals for use in daily medical practice and biomedical research.
Our facility
- A Siemens Eclipse HP and an IBA Kiube cyclotron
- Total floor area of 1400 square meters
- Three GMP-qualified preparation rooms
- QC labs for the testing of radiopharmaceuticals
- Logistic areas for the distribution of radiopharmaceuticals
- A radionuclide lab for preclinical preparations and other research activities

Cyclotrons
- Siemens Eclipse HP and IBA Kiube
- Deep underground in a bunker with 60 cm thick concrete
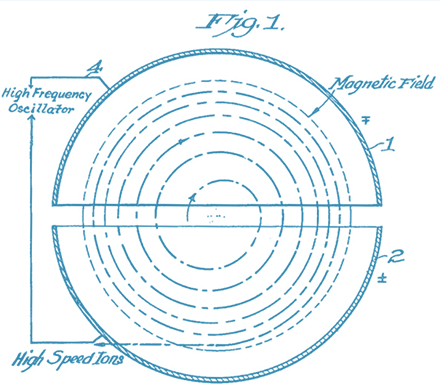
- The first cyclotrons were developed by the physicist Ernest Lawrence in 1929
- Accelerates electrically charged particles to a speed of over 165 million kilometres an hour
- The high speed causes nuclear reactions when colliding into other particles, creating radionuclides
- We link these radionuclides to molecules, creating radiopharmaceuticals
- These radiopharmaceuticals are used for diagnostics, treatment and research
RTM from the start
2001
The possibilities for a cyclotron facility in Nijmegen are evaluated for a first time. Plans are shelved for the time being.
2006
Ambitions flare up again. A cyclotron facility is considered an important missing link in the translational chain.
2010
A business plan is made. This is the basis for the application for a subsidy from the European Regional Development Fund.
2011
The subsidy is awarded and Radboud Translational Medicine is founded on August 18.
2014
Construction activities commence in October.
2015
On August 14, construction is finalized. Minister Schippers (Ministry of Health, Welfare and Sport) officially opens the facility on December 9.
2016
In October, the GMP-certificate is awarded by the Health Care Inspectorate.
The first deliveries of 18F-FDG to the Radboudumc take place in December.
2017
March: deliveries of 18F-FDOPA start for a clinical study.
November: deliveries of Ammonia start. In that same month, a new GMP-certificate is awarded.
2018
May: RTM starts processing QuiremSpheres (dispensing and final QC).
July: RTM receives a marketing authorization for 18F-FDG.
Delivery of 18F-PSMA-1007 for a clinical study starts.
RTM and Telix start a collaboration for the delivery of 89Zr-DFO-Girentuximab.
2020
May: permission is granted by the Health Care Inspectorate for producing and delivering 18F-NaF after submitting doctor's statement
July: 18F-DPA-714 is produced for a clinical study.
2021
18F-FE-PE21-E is produced for a clinical study.
2022
September: hoisting of the second cyclotron (IBA Kiube).
2023
April: second cyclotron, IBA Kiube, is operational
2024
In June RTM was added as a distributor for NeuraCeq® then in July for Radelumin® in the Netherlands.
Nuclear diagnostics
Nuclear medicine is a dynamic discipline and development is still in full swing. The availability of better cameras and software has led to an impressive improvement of the possibilities for nuclear diagnostics.
With the use of new radiopharmaceuticals, better visualization of disease processes is possible. Doctors can make better diagnoses and better choices when it comes to the various treatment options.
In this way, nuclear medicine delivers an important contribution to the development of the ‘personalized medicine’ concept, a customized treatment for every patient. This prevents the ineffective use of means, saves the patient from undergoing severe treatments and ultimately improves the treatment outcomes.
Positron emission tomography (PET) is also increasingly used in the development of new medicines. This technique can provide a valuable contribution to the research to elucidate the cause of diseases like many cancers, Alzheimer’s disease, Parkinson’s disease and diabetes.


Subsidy awarded by ERDF
On June 23, 2011, the Management Authority of the Provinces of Gelderland and Overijssel awarded a subsidy with a maximum amount of € 8,2 million euro from the European Regional Development Fund (ERDF) to the Consortium Translational Medicine (CTM, collaboration between Radboudumc and Radboud Translational Medicine) for the realization of a new cyclotron facility.
ERDF aims to strengthen the local knowledge economy, the regional innovative power and the competitive position of companies in the region. The ultimate goal is to improve the position of the eastern Netherlands as center of expertise for translational research. The initiative strives to stimulate the creation of opportunities for innovative initiatives and the collaboration between companies and knowledge institutes.
Interested in our products?
Contact us to discuss the possibilities.





































