Cooperation RTM-Alliance Medical
It is with great pleasure that we announce our cooperation with Alliance Medical Group (AMG) regarding the marketing, sales and distribution of our PET-radiopharmaceuticals in the Netherlands and Belgium. We have received our marketing authorization and Fludeoxyglucose (18F) RTM 200 MBq/ml will become commercially available in the Netherlands in October, with roll-out to Belgium and Germany planned in 2019. Lars Perk, Managing Director RTM: ‘Obtaining the marketing authorization in a relatively short period of time is an important milestone for Radboud Translational Medicine (RTM). Construction of our facility was completed in December of 2015 and we have made great strides in the three years since then. Now, we are ready for the next step: delivering FDG to a wide range of hospitals in the Netherlands’.
AMG will assist RTM in this new endeavor. AMG, a member of the Life Healthcare Group, is recognized as a valued partner for health systems providing essential and affordable diagnostic imaging services along with the manufacture and distribution of radiopharmaceutical products with a vision to be a market leading, internationally diversified healthcare provider focused on delivering sustainable, high quality and cost effective healthcare. AMG is Europe’s leading independent provider of imaging services, delivering medical imaging services in UK, Germany, Ireland, Italy, Spain, Norway, Finland and the Netherlands.
Radboud Translational Medicine has a state-of-the-art cyclotron facility and is committed to the development of PET-radiopharmaceuticals for routine medical use and clinical studies.

Other news
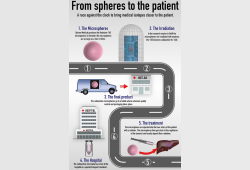
First liver cancer patient treated with microspheres irradiated in TU Delft
Radboud Translational Medicine will perform the dispensing of below product. The production and dis...
First Patient Dosed in Phase II Study of TLX250-CDx in Triple-Negative Breast Cancer
The below product is manufactured at RTM First Patient Dosed in Phase II Study of TLX250-CDx in Tri...

LinkedIn post Isologic goes electric
🔌Isologic Goes Electric! We are excited to announce the expansion of our electric fleet with the a...

Huntington’s Disease: A Review of the Known PET Imaging Biomarkers and Targeting Radiotracers
Klaudia Cybulska (postdoc radiochemistry) has written an article about Huntington's Disease. Abstra...
Interested in a collaboration?
Contact us to discuss the possibilities.





